Vocabulary Review Matter and Molecules Matter Molecules Isotopes Compounds Ions

Molecules are the essential building blocks of life. Without water molecules, life on Earth would not exist. All living organisms need poly peptide molecules for construction and part. Therefore an understanding of how atoms combine to grade molecules is fundamental to agreement the origins of life.
In your form
Download instructions, a grid and prompt cards to play atoms, molecules and ions 4-in-a-line as MS Word or pdf.
Download instructions, a grid and prompt cards to play atoms, molecules and ions four-in-a-line from the Pedagogy in Chemistry website: rsc.li/2WayTn8
What students need to know
Atoms, molecules and ions are all examples of particles that students might meet at eleven–14. But these terms are often used incorrectly in the media and everyday linguistic communication leading to students of all ages beingness confused equally to which is the correct term to utilise.
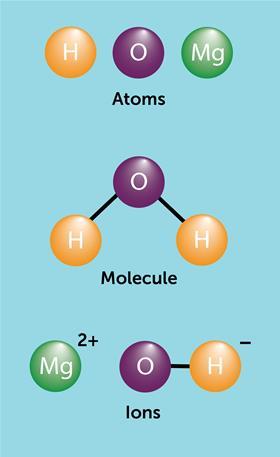
Students should understand that:
- Particles tin be atoms, molecules or ions.
- Atoms are single neutral particles.
- Molecules are neutral particles made of 2 or more atoms bonded together.
- An ion is a positively or negatively charged particle.
Ideas for your classroom
The thought of the globe beingness made of tiny particles is an aboriginal 1. You could kickoff the exploration of atoms with the ideas of Democratus (400 BC), who believed that all matter in the universe was made upward of tiny, indivisible, solid objects. He called these objects atoma or 'indivisible units'. At the kickoff of the 19th century Dalton plant bear witness to support Democratus' theory and proposed atoms to be solid spheres. Different spheres made up the unlike elements.
One of the fundamental problems for students learning virtually atoms, is that atoms are small-scale. Really, really small. This makes it difficult for students to conceptualise atoms as they cannot be seen, or touched, or investigated directly. A good starting point to introduce atoms and illustrate their pocket-sized size is to enquire students to break upwards a slice of graphite (the chemical element carbon) into as many pocket-sized pieces as they can. No matter how many pieces the students interruption the graphite into, they will never get a unmarried carbon cantlet. You can challenge higher attaining students to measure the size of an private cantlet using this experiment from Practical physics.
One of the key issues for students learning almost atoms, is that atoms are pocket-sized. Really, really minor. This makes it difficult for students to conceptualise atoms as they cannot be seen, or touched, or investigated directly. A skilful starting point to introduce atoms and illustrate their small size is to ask students to pause upwards a piece of graphite (the chemical element carbon) into as many small pieces as they can. No matter how many pieces the students break the graphite into, they volition never go a unmarried carbon atom. You tin can challenge higher attaining students to measure the size of an individual cantlet using this experiment from Practical physics (flake.ly/2Km5cgt).
When atoms combine, molecules are formed. For a few elements, when atoms of that element combine, a molecule of that element is formed eg Hii and Otwo. When atoms of some different elements combine, a molecule of a compound tin form, eg H2O. How to teach elements and compounds, in the eleven–14 serial, describes unlike strategies for teaching elements and compounds and the common misconceptions students may hold.
When atoms combine,molecules are formed. For a few elements, when atoms of that element combine, a molecule of that element is formed eg H2 and O2. When atoms of some different elements combine, a molecule of a compound tin can course, eg HiiO. How to teach elements and compounds (rsc.li/2W6MKut), in the eleven–14 series, describes different strategies for teaching elements and compounds and the common misconceptions students may hold.
Particle diagrams tin be used to help the students visualise the deviation betwixt an atom, a molecule of an element and a molecule of a chemical compound. In fact even Dalton in the 1800s proposed a series of diagrams to stand for the elements and compounds known at the fourth dimension. Use of color helps to distinguish between the cantlet types farther. Venn diagrams assistance students organise their agreement of the different particle types, as described in Atoms, elements, molecules, compounds and mixtures.
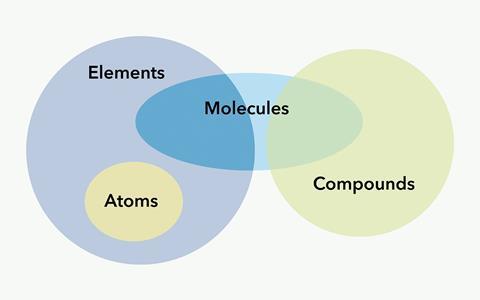
Particle diagrams tin can be used to assist the students visualise the difference between an atom, a molecule of an element and a molecule of a compound. In fact even Dalton in the 1800s proposed a series of diagrams to represent the elements and compounds known at the fourth dimension (Figure 1). Employ of colour helps to distinguish between the cantlet types further. Venn diagrams help students organise their agreement of the different particle types, equally described in Atoms, elements, molecules, compounds and mixtures (rsc.li/2wzLsxS).
An atom or a molecule can lose or gain electron(s) to form an ion. At this level students only need to know that an ion is a positively or negatively charged particle. All the same information technology may be worth introducing students to the electron at this signal. When an atom/molecule gains negatively charged electron(south), a negative ion is formed. When an cantlet/molecule loses negatively charged electron(southward), a positive ion is formed. This latter bespeak is something students often struggle with after on in their studies. Introducing the electron now, earlier students meet the other sub-atomic particles, can help to embed the thought that the loss of electrons results in a positively charged ion, and may help reduce confusion after on.
Owing to the interweaving of the terms cantlet, ion and molecule when describing the different particles, information technology is unsurprising that students become confused. Using games and an element of competition tin be helpful to bring some variety to the necessary student exercise. One such game is based on the classic Connect 4 game. You can download instructions, an example grid and game cards below.
Mutual misconceptions
As the students develop their agreement of chemic bonding farther, information technology is common for students to refer to ionic compounds equally molecules or to refer to intermolecular forces when explaining properties of ionic compounds. To avert these misconceptions, it is of import to introduce, and emphasise, the correct use of the terms ion and molecule from early on in a educatee's chemical studies.
A molecule is a neutral particle, composed of a fix number of atoms bonded together. The particle of the substance is the molecule, rather than the atoms that make upwards the molecule. By contrast, ionic compounds are fabricated up of an indeterminate number of ions, in a stock-still ratio. The particle of the ionic substance remains the ion. Using hands-on models tin assistance students with these tricky concepts – eg TIMSTAR MO84200 for molecules and Molymod MKO-127-27 for ionic structures. You can further explore the utilize of chemical models and their limitations in Using molecular models and in the vii simple rules to for science teaching serial.
A molecule is a neutral particle, composed of a set up number of atoms bonded together. The particle of the substance is the molecule, rather than the atoms that brand up the molecule. By contrast, ionic compounds are made up of an indeterminate number of ions, in a stock-still ratio. The particle of the ionic substance remains the ion. Using easily-on models can aid students with these tricky concepts – eg TIMSTAR MO84200 for molecules and Molymod MKO-127-27 for ionic structures. You tin further explore the utilise of chemical models and their limitations in Using molecular models (rsc.li/2wAsOpA) and in the seven simple rules to for scientific discipline teaching series (rsc.li/2XmwHKr).
Other misconceptions students may hold are discussed in Beyond appearances: Students misconceptions about basic chemical ideas (rsc.li/2WBsd5L), including that atoms share the properties of the bulk textile and that molecules accept different properties in different states.
Progression to 14–16
At 14–xvi, students are introduced to sub-diminutive particles and how these define the nature of atoms and ions. Students then get on to study the difference between the nature of the forces that be betwixt atoms, molecules and ions, which they apply to explain the concrete properties of ionic and covalent compounds.
The resources, Why practice atoms class ions allows students to assess their understanding of atoms, ions and ionic compounds and enables the teacher to place any misconceptions.
The resource, Why do atoms form ions (rsc.li/2Kptsyq) allows students to assess their understanding of atoms, ions and ionic compounds and enables the teacher to place any misconceptions.
Have-home points
- Particles can be atoms, molecules or ions.
- The term molecule is oftentimes used incorrectly to refer to any type of chemic compound. A molecule is a neutral particle fabricated of ii or more atoms bonded together.
- Take intendance with your ain language, especially when referring to compounds formed during chemical reactions. Make the distinction betwixt each particle type explicit. Give students the opportunity to organise their agreement of the different particle types with Venn diagrams.
- A good understanding of the different particle types will help students when they report structure and bonding at 14–16.
![]() Catherine is head of science at Hinckley Academy and John Cleveland Sixth Form Heart, an eleven–eighteen academy in Leicestershire.
Catherine is head of science at Hinckley Academy and John Cleveland Sixth Form Heart, an eleven–eighteen academy in Leicestershire.
Source: https://edu.rsc.org/cpd/atoms-molecules-and-ions/3010574.article
Belum ada Komentar untuk "Vocabulary Review Matter and Molecules Matter Molecules Isotopes Compounds Ions"
Posting Komentar